New Clinical Partnership Announcement
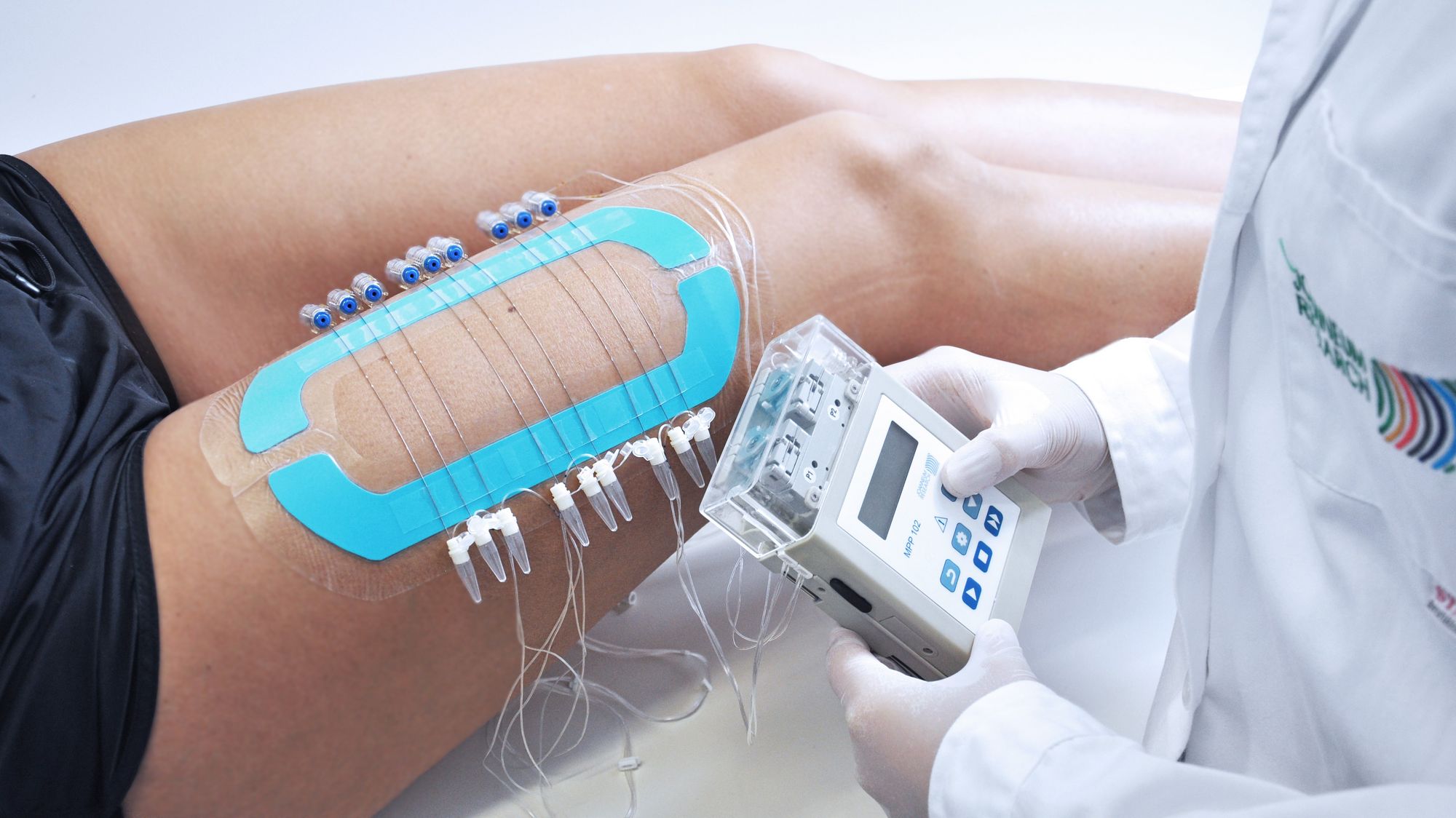
JOANNEUM RESEARCH Forschungsgesellschaft mbH and AXIS Clinicals LLC are excited to announce the first ever partnership for the clinical applications of dermal open flow microperfusion (dOFM™) technology.
We study molecular mechanisms of metabolic networks affected by diabetes, obesity & other diseases.
We support dermatological drug development from early stage candidate screening to late-stage clinical testing.
We provide data for drug development as well as unique insights into brain metabolism and signaling.

We conduct untargeted metabolomics studies and targeted quantification of metabolites to investigate metabolic processes.

We analyze and differentiate endogenous or therapeutic peptides and interfering products in (pre-)clinical study samples.

We determine metabolite turn-over rates, body water content, total energy expenditure as well as trace activity of metabolic pathways.

IVRT with Franz diffusion cells in combination with GLP compliant bioanalytical analyses.
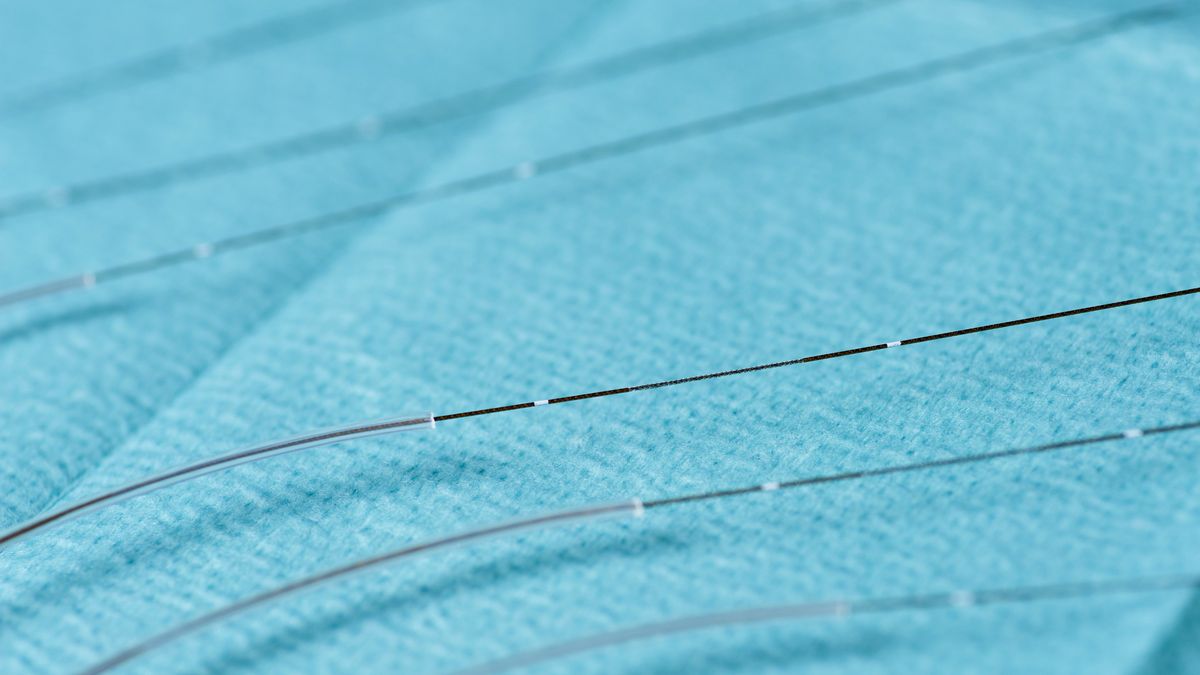
We investigate and compare skin penetration and release rates of topically applied APls.
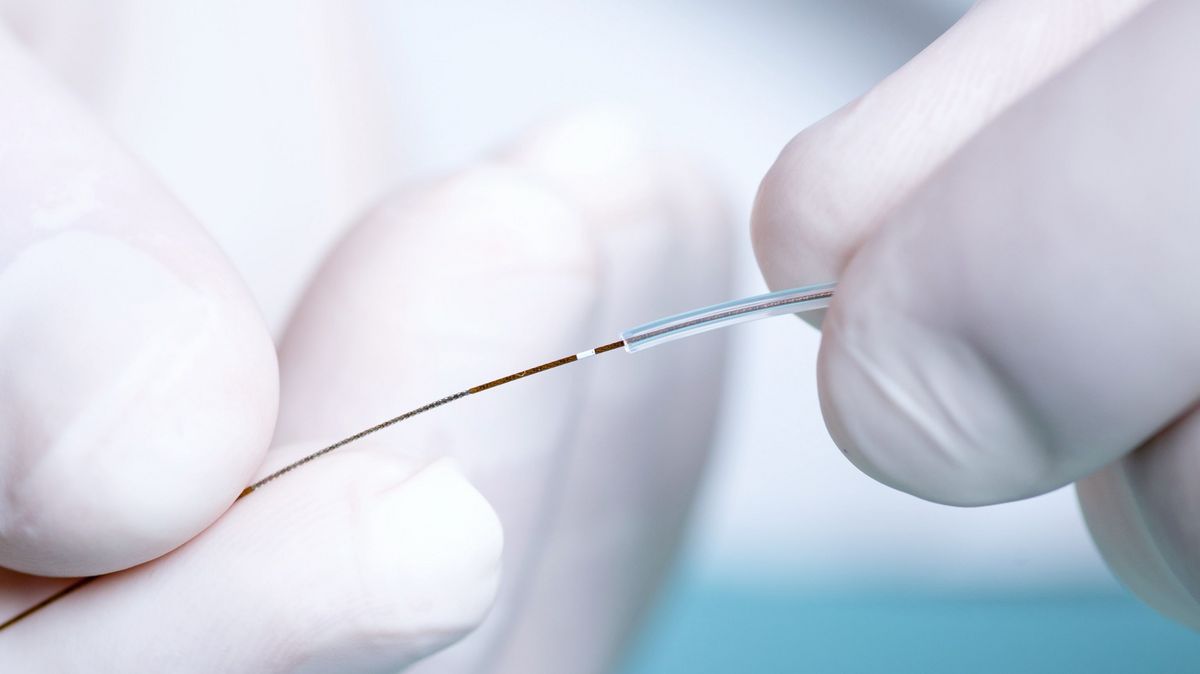
In-vivo preclinical assessment of intradermal PK/PD profiles in animal disease models.

Comprehensive data management services tailored to your clinical and preclinical studies.

Better interpretation of study results for comprehensive insight into your data by graphical data visualization.

Fully validated online system to capture case report forms (CRFs).

HEALTH – Institute for Biomedical Research and Technologies
Neue Stiftingtalstrasse 2/Entrance A/Third Floor
8010 Graz
Phone: +43 316 876-4000
Fax: +43 316 8769-4000